

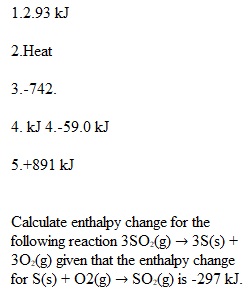
Q Question 1 1 / 1 pts How much heat (in kJ) must be added to a sample of this metal weighing 95.6 g for the temperature to change from 30.0 °C to 98.0 °C? Correct! Question 2 1 / 1 pts Joule is the SI unit of ________________. Question 3 1 / 1 pts The standard molar enthalpy of combustion of butane (C4H10) is –2877 kJ. What is the enthalpy change for the combustion of 15.00 g C4H10? C4H10(g) + 13/2 O2(g) ? 4 CO2(g) + 5 H2O(g) Question 4 1 / 1 pts Given the following reactions Fe2O3(s) + 3 CO(s) ? 2 Fe(s) + 3 CO2(g) ?H = -28.0 kJ 3 Fe(s) + 4 CO2(s) ? 4 CO(g) + Fe3O4(s) ?H = +12.5 kJ determine the reaction of Fe2O3 with CO 3 Fe2O3(s) + CO(g) ? CO2(g) + 2 Fe3O4(s) Question 5 1 / 1 pts Calculate enthalpy change for the following reaction 3SO2(g) ? 3S(s) + 3O2(g) given that the enthalpy change for S(s) + O2(g) ? SO2(g) is -297 kJ.
View Related Questions